what type of hybrid orbitals form when 2 atomic orbitals are mixed?
2s 2p. Hybridization corresponds with the electron domain geometry.
 |
| Solved Complete The Table Below For The Composition Of Chegg Com |
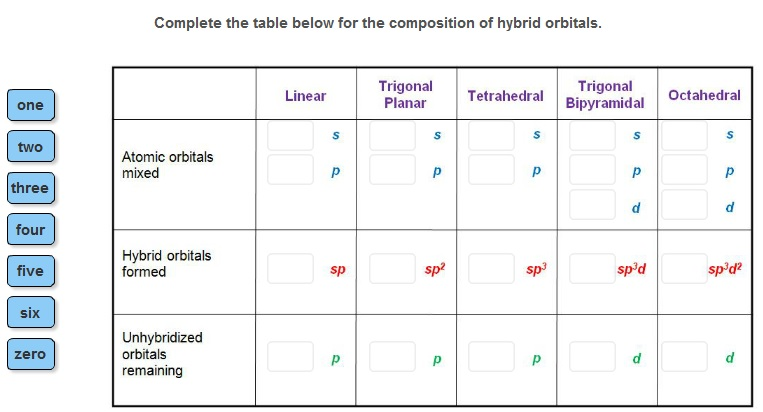
The number of hybrid orbitals in a set is equal to the number of atomic orbitals that were combined to produce the set.
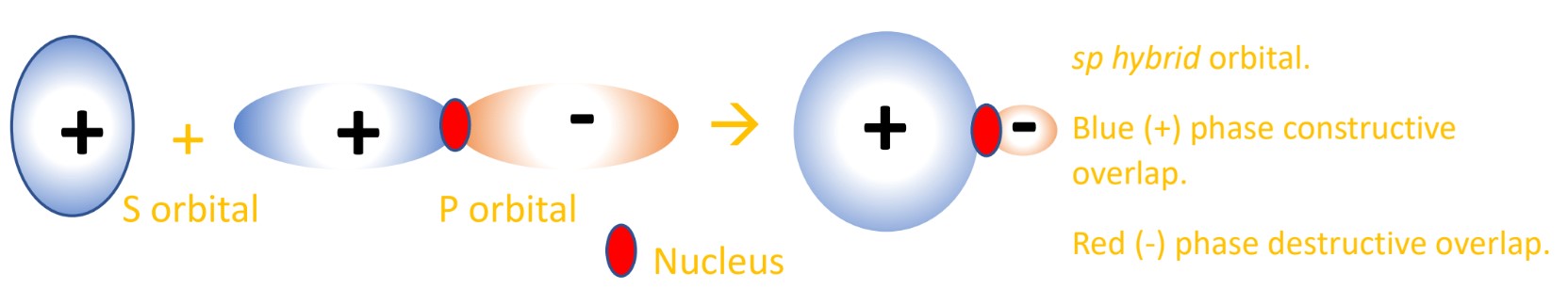
. A hybrid orbital is an orbital formed by the combination of two or more atomic orbitalsThe resulting orbital has a different shape and energy than the component orbitals that form it. Hybridisation is the process of mixing atomic orbitals of different shapes with approximately the same energy to produce the same number of hybrid orbitals with the same shape energy and. 2 Atomic orbitals can mix or hybridize in order to adopt an appropriate geometry for bonding. Thus the orbitals that point the correct directions are made by combining the 2s and all three of the 2p orbitals.
Pre-hybridization 1s 2s 2px 2py and 2pz atomic orbitals. The atomic orbitals of the central atom mix to form hybrid orbitals are one s and three p. Four hybrid orbitals In the current case. For example mixing an s and three ps give four new sp3 hybrid orbitals.
Solution for What type of hybrid orbitals form when 3 atomic orbitals are mixed. Nuclei contain neutrons and protons bound together by. How many hybrid orbitals does carbon have. The four valence atomic.
How many atomic orbitals are used to form hybrid orbitals. The Hybridization in which one S and two P orbitals of different shapes and energy mix to form 3 hybrid orbitals is known as SP 2 Hybridization. 2 SP 2 Hybridization. How many hybrid orbitals are formed when 2 atomic orbitals are mixed.
The number of new hybrid orbitals is equal to the number of AOs mixed together to form the hydrid orbitals. This arrangement results from. Hybridization of sp3d includes the mixing of orbitals of one s 3 p and 1 d to set up 5 sp3d hybridized orbitals of equivalent energy. They consist of trigonal bipyramidal geometry.
How many atomic orbitals are used to form hybrid orbitals. All orbitals in a set of hybrid orbitals are equivalent in shape and. An atom consists of two components a nucleus and its orbiting electrons. Each hybrid orbital is.
The four valence atomic orbitals from an isolated carbon atom all hybridize when the carbon bonds in a. An important one is the sp- hybridization where one s- and one p-orbital are mixed together. The sp 2 hybridization is the mixing of one s and two p atomic orbitals which involves the promotion of one electron in the s orbital to one of the 2p atomic orbitals. The valence orbitals of a central atom surrounded by three regions of electron density consist of a set of three sp 2 hybrid orbital and one unhybridized p orbital.
Group of answer choices. To get four new orbitals we have to combine four atomic orbitals. An orbital created by the combination of atomic orbitals in the same atom. Filled atomic orbitals mix to form three sp2 orbitalseach is of 33 s.
Are a type of atomic orbital that results when two or more atomic orbitals of an isolated atom mix the number of hybrid orbitals on a covalently bonded atom is equal to the.
 |
| Solved 1 2 Attempts Left Check My Work Be Sure To Answer All Chegg Com |
 |
| Solved Which Types Of Atomic Orbitals Of The Central Atom Mix To Form Hybrid Orbitals In A Mathrm Siclh 3 B Mathrm Cs 2 Mathrm C Mathrm Scl 3 Mathrm F Mathrm D Mathrm Nf 3 |
 |
| Logical Class Home |
 |
| A N Atom Orbitals And Their Linear Combination To Form N2 Molecular Download Scientific Diagram |
 |
| Solved Which Of The Following Are True Statements Regarding Chegg Com |
Posting Komentar untuk "what type of hybrid orbitals form when 2 atomic orbitals are mixed?"